The end of smoking. Period.
NoMore
NoMore will be the world’s first personalized FDA approved combination medical device focused exclusively on assisting individuals with smoking addiction therapy.
NoMore is a groundbreaking, innovative platform that uniquely addresses each of the core addiction attributes (Biochemical, Sensorial and Behavioral) to craft a dynamic and completely personalized user experience.
Role
Director of Engineering, Project Lead
Year
2020-2022
Company
10XBeta
Client
Predictably Human
The device couldnt meet the specs without having the following features:
Biometric security
Dose and concentration control algorithm
Ceramic heating/wicking elements
Safety mechanisms to prevent generation of harmful constituents
Puff topology sensing
Robust case, pen, cartridge architecture
Novelty in every puff
We conducted a comprehensive study of nicotine and its mechanisms, focusing on thermal aerosol dynamics and various puff topology profiles. This investigation extended to current heating and wicking solutions, flavor additives, and harmful constituents. We examined both passive and active mixing technologies, and developed scientific methods for their measurement in the laboratory setting. Additionally, we identified key parameters and data that could inform and drive the cessation model
• Tobacco consumption vector
• Tobacco consumption profile
• Puff topology
• Cessation objectives
• Medication profile
• Sleep pattern
• Co-morbidities
• Dietary profile
• Quit attempt history
• Nicotine Metabolite Ratio
• Age
• Sex
• Oral contraceptive usage
• Racial/ethnic profile
• Height
• Weight
• BMI (calculated)
• Adipose tissue proportion (calculated)
• Smoking index
• Years smoking
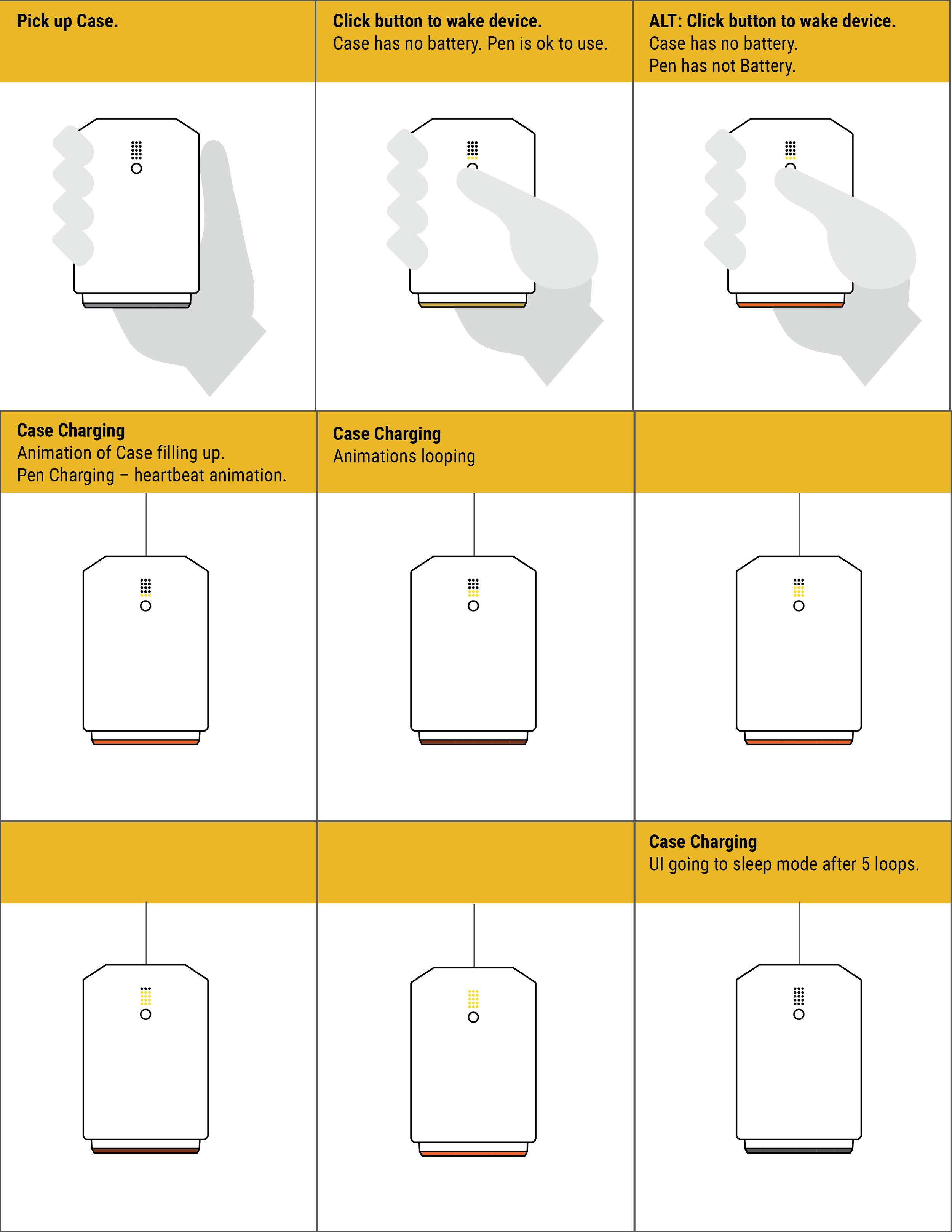
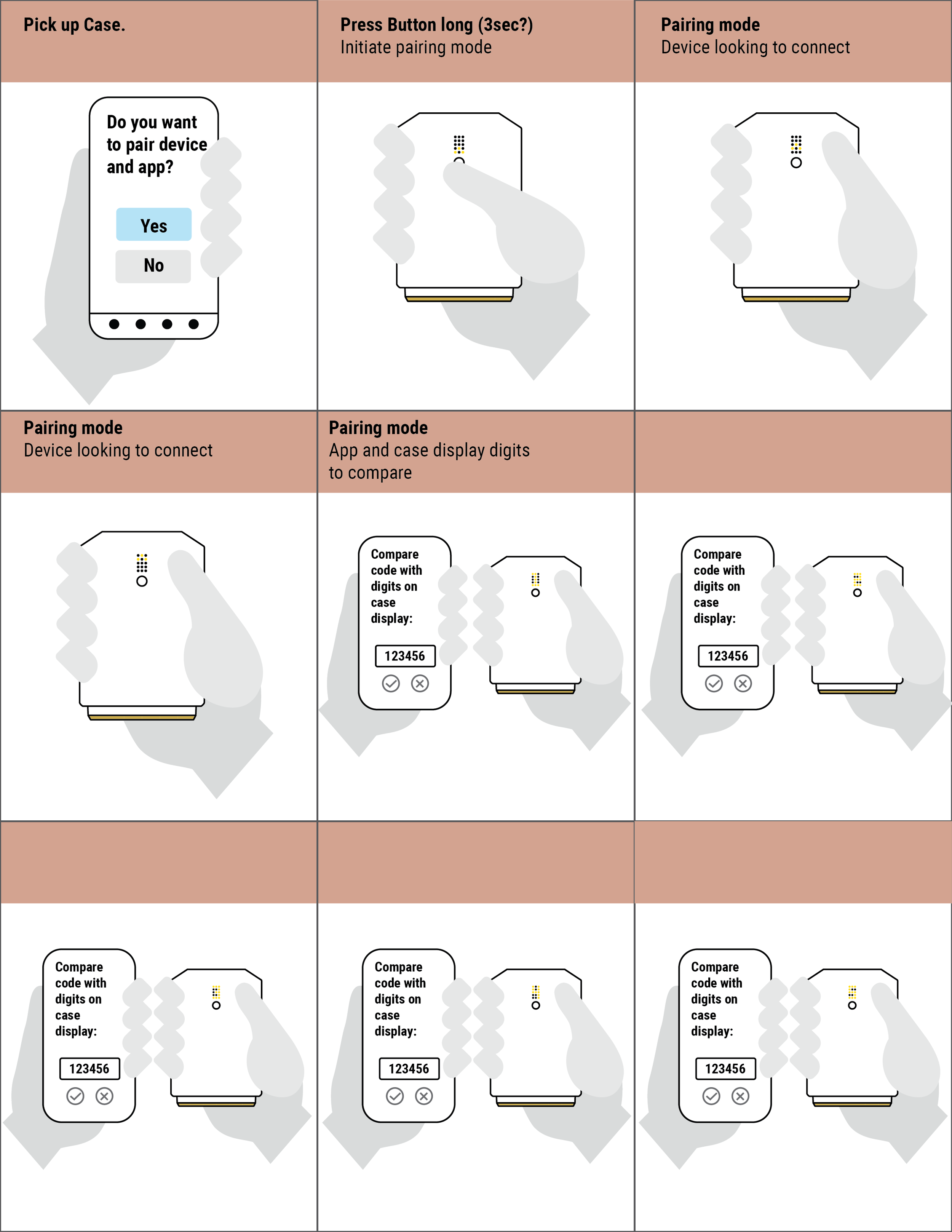
We thoroughly mapped all potential states, transitions, and outputs, which was essential for the development and testing of the device firmware, app software, and user interface implementation.